

PROJECTS
TomoWave Medical Group
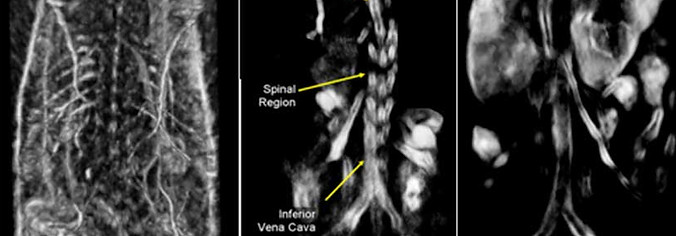
TomoWave Medical Group is dedicated to advancing and applying cutting-edge 3D/4D Photoacoustic Tomography (PAT) technology. This innovative technology enables structural, functional, and molecular imaging through its exceptional resolution, contrast, and sensitivity. It facilitates real-time, image-guided animal experiments and enables non-invasive, synchronous image-guided biopsies and surgeries. As a leader in the research, development, and production of photoacoustic tomography imaging equipment, TomoWave has successfully launched two pioneering product lines: the Preclinical Animal Photoacoustic Imaging System and the Clinical Photoacoustic Diagnostic System. Notably, the Preclinical Animal Photoacoustic Imaging System has achieved significant commercial success, with sales exceeding 3.5 million USD.
Torskal: Pioneering Green Nanomedicine for Cancer Therapy

Torskal is an innovative French biotech company pioneering non-ionizing plasmonic photothermal therapy (PTT) for oncology, leveraging a proprietary green nanotechnology platform. Headquartered in La Réunion, France, the company focuses on highly targeted, sustainable cancer treatments using plant-derived gold nanoparticles with superior biocompatibility and minimal environmental impact.
Torskal’s lead program, TSK-001, is designed for nodular basal-cell carcinoma (BCC), the most common form of skin cancer. The therapy employs near-infrared light to activate tumor-localized nanoparticles, generating precise, localized heat to eradicate malignant cells while sparing healthy tissue—offering a non-invasive, non-toxic alternative to radiation and chemotherapy.
Founded and led by Dr. Anne-Laure Morel, an expert in green nanochemistry, Torskal holds a robust patent portfolio granted in over 40 countries. The company is currently advancing toward Phase 1–2 clinical trials, aiming to translate sustainable photothermal therapy into clinical reality.
Services
International Investment and Financing Services:
-
Angel and Early-Stage investments
-
International incubation with equity holdings
-
Financing consulting
-
Investment matchmaking activities
-
Industrial Park connection services
International Market Access Consulting Services:
-
FDA agency services
-
International standard testing
-
QSR/cGMP declaration for medical device products
-
Medical device registration and clinical evaluation
Market Promotion and Sales:
-
Promotion and sales of biopharmaceutical and medical device products in North American and Asian markets
-
Establishment of a global online sales system
-
Operation of warehousing and logistics services in North America and Asia
Production Outsourcing and Global Procurement Services:
-
Process research and validation for products
-
Original equipment manufacturing for pilot and post-market stages of products.
-
Procurement and customized procurement services for medical devices and research experimental products.



